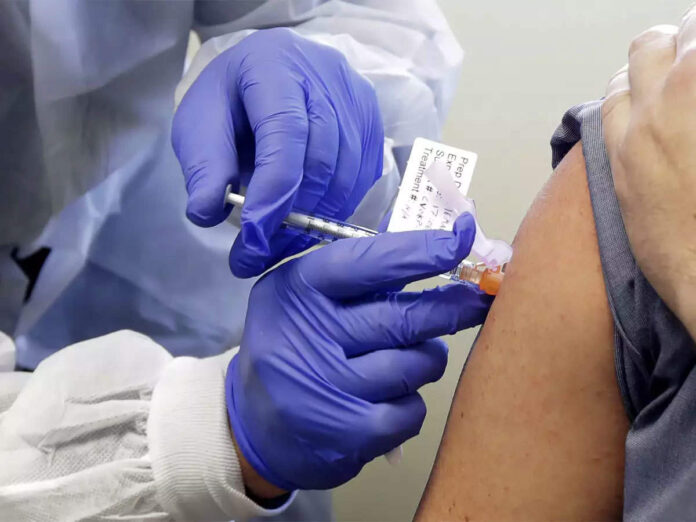
White House officials have reportedly blocked new federal guidelines for the emergency release of a coronavirus vaccine, including one that would likely ensure that no vaccine could be authorized before the November 3 presidential election, the New York Times reported on Monday.
The US Food and Drug Administration submitted the guidelines to the Office of Management and Budget more than two weeks ago, but they stalled with White House Chief of Staff Mark Meadows, the Times reported, citing people familiar with the approval process.
An administration official told Reuters that the approval process was still pending and denied any Election Day connection.
The FDA is seeking other avenues to ensure that vaccines meet the guidelines, the Times reported.
A main point that has been the recommendation that volunteers who have participated in vaccine clinical trials be followed for about two months after the final dose before any authorization is granted, reported The Times.





